Conformations of MreB protein interacting with GroEL/ES and TRiC chaperonins
Chaperonins ensure correct functional folding of proteins in cells. Despite their crucial role, their mechanisms of action are still open to questions. In a recent Scientific Report study “Differential conformational modulations of MreB folding upon interactions with GroEL/ES and TRiC chaperonins”, we investigate the action of GroEL/ES and TRiC systems to fold MreB substrate protein.
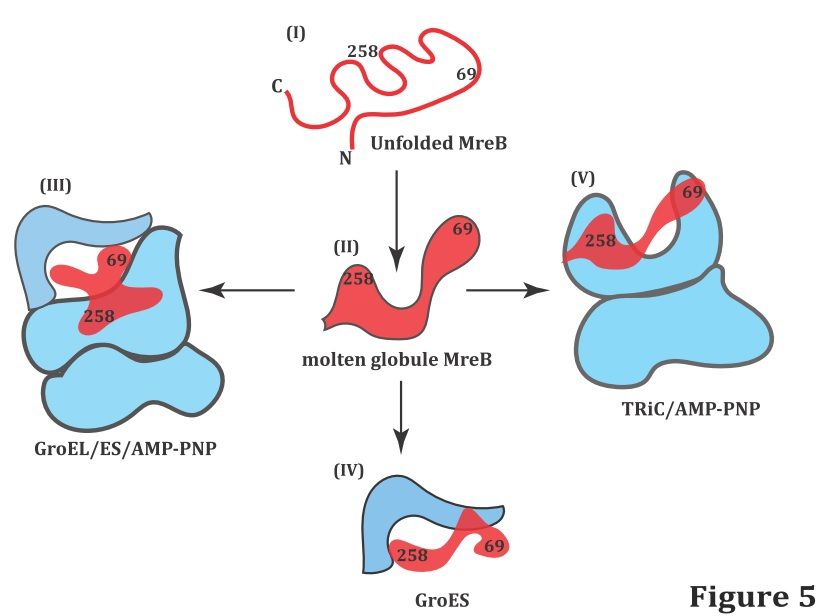
MreB is a homologue to actin in prokaryotes both structurally and functionally, and plays a central role to control cell shape, division or locomotion. The strength of our approach is to take advantage on complementary time-resolved fluorescence techniques (FCS, anisotropy and FRET) to monitor the conformational rearrangements of MreB occurring in GroEL/ES and TRiC assisted refolding.
Significance:
- We clearly establish that MreB forms complexes with TRiC, GroEL and GroES independently and in concert, and we quantify the complexes sizes and dynamics.
- We demonstrate an unexpected role of GroES acting as an unfoldase to induce a dramatic expansion of MreB and facilitate refolding in the GroEL/ES system. Our analysis importantly provides quantitative distance information about the MreB conformation expansions for both GroEL/ES and TRiC systems.
/image%2F1383851%2F20240412%2Fob_d06231_figtoc-aunc.jpg)
/image%2F1383851%2F20240410%2Fob_983d9d_s3ic-2024-banner-1920x380.png)
/image%2F1383851%2F20231116%2Fob_7c6f32_untitled16-figtoc.jpg)
/image%2F1383851%2F20231115%2Fob_6016e9_imagecombo.jpg)



